Analysis sent out every Monday
Build with Axial: https://axial22.axialvc.com/
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company . We are excited to be in business with you - email us at info@axialvc.com
Fluorescent dyes for microscopy
Microscopy has been a driving force for breakthrough discoveries in biology from how cells divide to how proteins interact with each other. And it has historically attracted unique characters over the centuries like Antonie Van Leeuwenhoek, the Janssens, and Douglas Prasher. The field has undergone rapid acceleration over the last 2 decades with the advent of super-resolution microscopy, which allows imaging of the cell beyond the diffraction limit of light. A key figure here is Luke Lavis who develops fluorescent dyes. Improvements in super-resolution microscopy depend on new & better dyes requiring a diverse catalog of colors and very bright & long-lasting imaging probes. Lavis and the group he leads combines rare expertise in both chemical synthesis and dye design. His work shows the value of building catalogs of imaging dyes to enable new types of screens.
“I sometimes call Luke our secret weapon here. Every advance I’ve made in my career has been due to fluorescent probes.”
- Eric Betzig, Nobel Prize Laureate in Chemistry for 2014
Lavis is the head of the molecular tools and imaging group at HHMI’s Janelia Research Campus (i.e. Janelia Farm), a place where tool builders work side-by-side fundamental researchers. With superstars like Brian English and Jonathan Grimm among many others part of the group, Lavis has been able to lead the way in the design & synthesis of new fluorescent probes enabling new ways to image the cell. A chemist by training, Lavis’ probes have given superpowers to the physics of microscopy leading to new biological discoveries. In accordance with the holiday season, Lavis is like Santa Claus for imaging dyes. Growing up in Oregon in a hippy household but also working in industry prior to starting his academic career, Lavis brings the values of generosity & usefulness to his work. His group is an open-source hub for dyes, sending out free vials to hundreds of labs around the world.
On the commercial side, innovation in microscopy and imaging dyes is leading to new ways to measure protein motion and discover new drugs for previously undruggable or hard-to-drug targets. Eikon Therapeutics, founded by Betzig and Robert Tjian, has taken the lead to commercialize super-resolution microscopy for drug discovery with the thesis that live-cell protein motion is more correlated to human physiology. There are also interesting opportunities to develop new sensors, combining dye design and protein engineering, for the brain as either a diagnostic for neurodegeneration or a research tool for something like neural tracing. Finally, there is a need for catalogs of dyes to expand access to these tools - think Addgene for chemistry (i.e. Addchem).
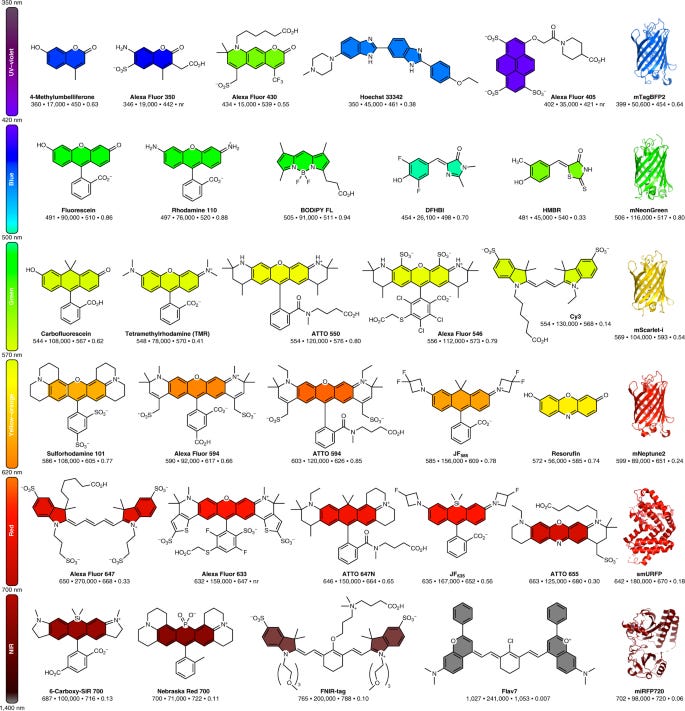
Fluorescent dyes are compounds that absorb light and emit it at a higher wavelength. This creates fluorescence in various colors depending on the wavelength shift. These dyes can be organic molecules like rhodamine or fluorescein but biological fluorophores like green fluorescent protein (GFP) are used too. GFP created a revolution itself in the 1990s as a quick way to track proteins. However, they often tend to aggregate, have lower diffusion rates, and are not as bright as organic dyes. On the other hand despite being less clunky than GFP, organic dyes have historically been pretty tough to synthesize. Starting in 1887, fluorescent rhodamine dyes began to get synthesized. However, their use in biology was limited for over a 100 years due to the difficulties to chemically modify rhodamine backbones and create variants for different brightness and colors. In 2011, Grimm and Lavis brought 21st Century chemistry to rhodamines and discovered a new way to use palladium-catalyzed chemistry for rhodamine synthesis. Then in 2015, the Lavis group invented a general structural modification (i.e. azetidine ring) to increase the brightness & photostability of dyes while maintaining their cell permeability. The Janelia Fluor series of dyes came out of this work and released the floodgates to do previously unimaginable experiments in single molecule imaging - capturing a crisp image of a protein in just a millisecond versus >10 ms previously. A successor paper in 2017 built on this research to systematically analyze modifications of rhodamines. This unlocked a way to build a catalog of dyes that cover the entire spectral range (from blue to far-red) and figure out ways to synthesize these dyes in a single-step with lower-cost inputs. This 10-year era had 3 main arcs:
2011 = tinkering with old rhodamine dyes by adding new side groups and ways to link them to target proteins to make the dyes brighter and more photostable. In particular, the Lavis group found ways to keep dyes in their excited state for a little longer and not go back to a ground state.
2015 = the key breakthrough in imaging dyes where the Lavis group unveiled rhodamine dyes that were ~10x brighter than past versions
2017 = establishing a set of design principles (linking structure to spectral properties) to fine tune rhodamine dyes to generate any color you want. With a rational framework in place, a chemist can synthesize dyes that are more cell permeable or photostable, can cross the blood-brain barrier, water soluble, and so much more.
With all this progress on the design side, new ways to attach dyes to proteins in a cell are needed. The main labeling strategies are (1) amine- and thiol-reactive fluorophores, (2) click chemistry, and (3) alternative hybrid and non-covalent approaches. To use the full palette of dyes, labeling chemistry has become the new problem to sort out.
Eikon is the first-mover to use this toolkit for drug development. In combination with super-resolution microscopy & figuring out ways to automate them (i.e. Bender), the company is building a platform to measure single-molecule movement of proteins in living cells. Something that cannot be done with traditional imaging assays. The ability to screen chemical compounds for their effects on protein activity in live cells generates a unique dataset to discover new, or even better ignored, chemical matter that inhibit or promote protein interactions. To explore new investment ideas, I often just ask - what does Eikon Therapeutics enable? The company’s platform collapses several screens into one to find hits that modulate PPIs. On the tools side, ONI Bio is commercializing a benchtop device. And more is in development. However, talent and expertise with the microscope(s) & dyes are scarcer than the actual tools. A grad school project of mine relied on a lattice-sheet microscope, the only one of the West Coast at the time. Despite the device costing millions of dollars, the crew operating it were immensely more valuable - they were a team of 3 who would smoke cigarettes in between Barker Hall and LKS. Although I wouldn’t smoke, I would hang around to hear their funny stories: 2 became professors and one went on to work at Insitro. With access to this talent through Tjian & Betzig, Eikon has a significant short-term advantage that they will likely use to establish various partnerships and develop a pipeline. I’ve always comped Eikon to Relay Therapeutics. But once Eikon does get committed to clinical assets, a second-generation of companies will likely emerge to convert data on live-cell protein motion into new medicines.
But this all comes back to dyes. Sooner-or-later, but probably later due to a lack of high enough economic demand to drive down costs, super-resolution microscopes will become commoditized. Even if they are only within the grasp of a few companies, they will increasingly differentiate themselves on dye design and their applications. For example, some versions of super-resolution microscopy (PALM) rely on photoactivatable caged imaging agents that are much harder to synthesize than conventional dyes. The quickest way to improve the resolution of these methods is to invent better dyes. Even incremental progress on the dye side could lead to leaps for microscopy and biology.
Dyes plus microscopes allow us to peer into the choreography inside the cell. I remember after seeing an animation of the cell from Rob Lue my freshman year, I’ve always thought of it similar to a nightclub, maybe like Studio 54. Well over full capacity but there seems to be some level of order. And by color coding the cell with dyes, various parts of the cell are being mapped out and our understanding of its order is coming into full view. Progress in dyes over the last decade has accelerated progress in microscopy helping move from fixed to live cells, cell lines to in vivo, and finally beyond the diffraction limit of light. A compelling company can emerge that creates an ecosystem around an open-source catalog of dyes and brings new tools & chemistry to develop new drugs, diagnostics, and tools. You could imagine a Sears-like catalog of compounds classified by their extinction coefficients/quantum yields and their absorption/emission maximums. Partners pay a recurring access subscription along with long-term product economics.
What technical work is left to be done?:
Labeling strategies: as previously touched upon, new chemistry to connect dyes to proteins are needed. This would expand the types of things that can be imaged in a cell.
Hybrid sensors: there is exciting research ongoing to build hybrid small-molecule protein sensors that combine the cell specificity of fluorescent proteins with the spectral advantages of dyes.
Chromatography: purifying dyes is the current bottleneck. Finding ways to reduce rounds of chromatographic purification can lower costs substantially (want to get to dollars per milligram) and increase yield. Connected to this, simpler synthesis/purification methods can make distribution of dyes easier. Compared to genetically-encoded imaging tools that just require shipping DNA, access to fluorescent dyes is often limited by the need for expert chemists and multi-step syntheses.
These problems are being solved. As we begin to realize the value of higher-resolution imaging of the cell with new medicines and discoveries, microscopy and dyes will become a more central part of work in biology.