Analysis sent out every Monday
Axial: https://linktr.ee/axialxyz
Axial partners with great founders and inventors. We invest in early-stage life sciences companies such as Appia Bio, Seranova Bio, Delix Therapeutics, Simcha Therapeutics, among others often when they are no more than an idea. We are fanatical about helping the rare inventor who is compelled to build their own enduring business. If you or someone you know has a great idea or company in life sciences, Axial would be excited to get to know you and possibly invest in your vision and company . We are excited to be in business with you - email us at info@axialvc.com
N-of-1 trials
N-of-1 trials focus on understanding the effects of various interventions for an individual patient. In 1986, a group out of McMaster University published the first paper defining N-of-1 trials inspired by a drug trial for asthma - https://www.nejm.org/doi/pdf/10.1056/NEJM198604033141406 In this type of trial, a patient alternates between taking a drug and placebo. These treatments can be sequenced in various ways to establish the effects of the drug. After the trial, statistical analysis tools can be used to determine the efficacy of the drug and the one trial can be aggregated with other N-of-1 trials to infer the drug’s efficacy across a population.
N-of-1 trials are valuable for their ability to capture intra-individual variation. Other trial designs are good for inter-individual diversity and meet their limitations where patient responses to an intervention have high variation. N-of-1 become useful in situations where a randomized clinical trial (RCT) cannot address:
Diseases with low prevalence (i.e. rare diseases)
Diseases with large response variability (i.e. chronic pain)
Patients who have been on long-term treatment regimens
Patients who are medical outliers
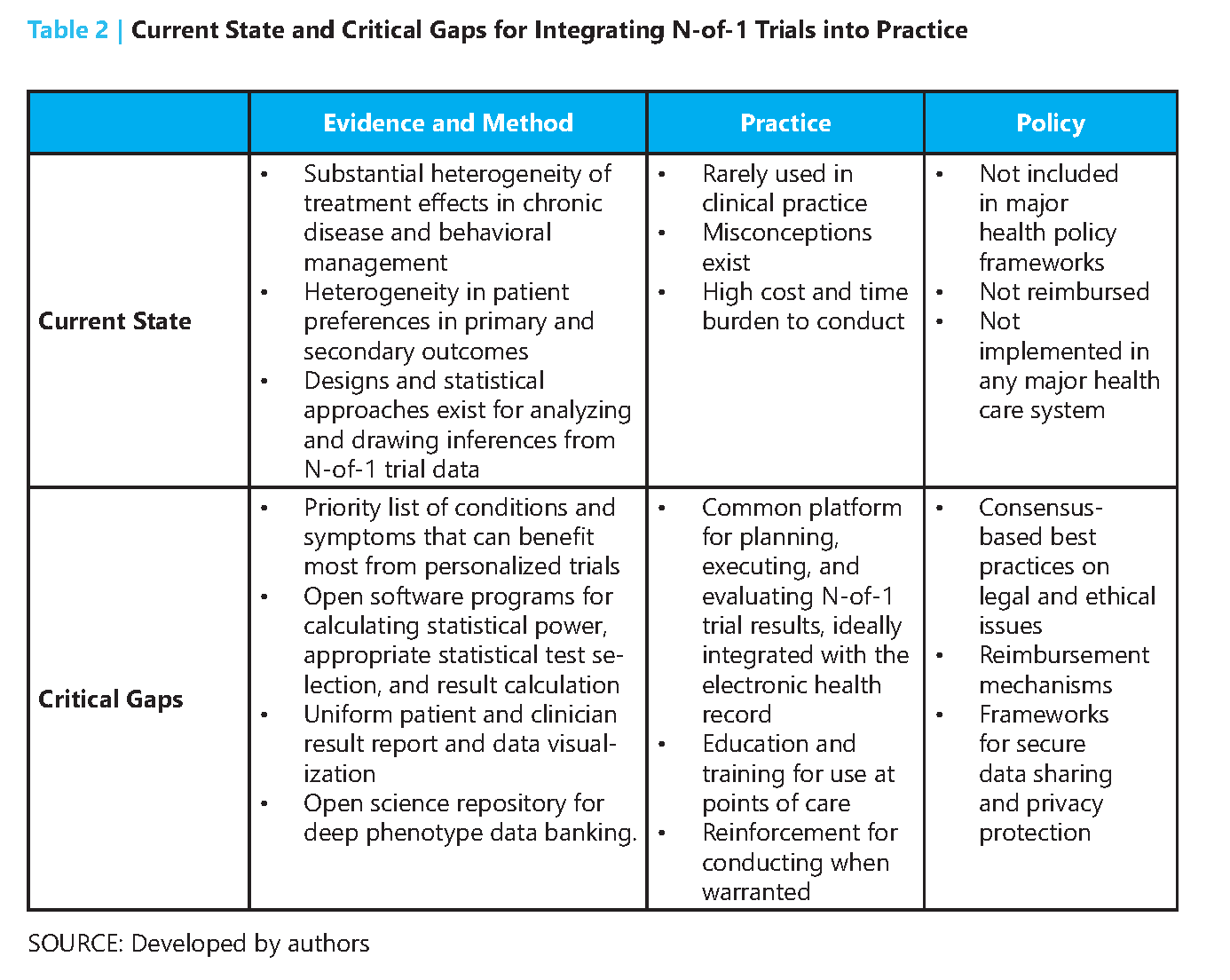
In short, N-of-1 trials can make drug development for rare diseases feasible where thousands have no approved medicine. Moreover, N-of-1 trials can act as an early signal on where to engage in a costly RCT and identify predictors of response beforehand. Ensuring trial quality across patients and comprehensive reporting can help N-of-1 trials to gain more acceptance from the medical community. Two large opportunities are to bring the power of antisense oligonucleotides (ASO) to N-of-1 trials (invest upfront and costs go down overtime) and build better software/data tools to capture data from these trials and put them into an EHR so results can be aggregated and analyzed with confidence by clinicians.